Abstract
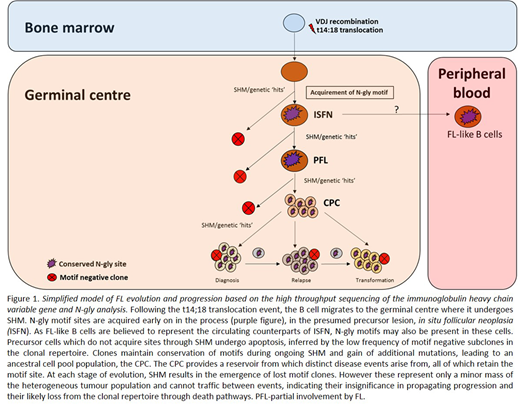
Introduction: Follicular lymphoma (FL) cells retain expression of a functional B cell receptor (BCR) despite the loss of one Ig allele due to the hallmark t14:18 translocation and ongoing somatic hypermutation (SHM) of the variable genes (V genes) which increases the likelihood of crippling mutations. SHM introduces N-glycosylation (N-gly) motifs within the V genes, a feature exclusively restricted to germinal centre (GC)-derived lymphomas. Oligosaccharides of the high mannose type are added to motifs and interact with calcium-dependent lectins associated with cells of the microenvironment. This activates the BCR signalling pathway and likely contributes to the survival, retention and proliferation of tumor cells in the GC. Determining at what stage of disease evolution N-gly motifs are acquired and their behaviour during progression can ascertain their importance in pathogenesis and their potential as an effective therapeutic target. To achieve this, we analysed motifs within the Ig heavy chain variable gene (IGHV) of tumor-related subclones across temporal FL samples.
Method: Genomic DNA from three FL patients taken at different time points of disease progression were analysed. In total, 8 samples were selected, all carrying an IGHV3 rearranged tumor clone. IGHV DNA amplicons were sent for 2x250bp paired-end sequencing using the Miseq Illumina platform (Genewiz, NJ). Tumor-related reads with counts greater than ten were selected following analysis on IMGT/HIGH-V-QUEST. Reads were aligned and unique sequences were assigned as subclones. Additional tumor related reads sequenced on the Roche 454 Life Sciences Genome Sequencer FLX were available (Patients 4 & 5). Subclones were analysed for N-gly motifs and evolutionary pathways were generated using the IgTree program, based on intraclonal SHM profiles and homology of tumor clones to the germline IGHV sequence.
Results: The earliest time point samples for Patient's 1, 3, 4 & 5 contained one N-gly site within the IGHV of the MC defined by the largest count number. These sites were conserved in >97% of unique subclones (p<0.0001) despite variations in the nucleotide sequence within the region as a result of ongoing SHM. This conservation included the most mutated subclones which had a mean SHM rate of 16.56%. Conservation was maintained across disease events. Patient 2 contained four N-gly sites located within the CDR1, FR2, CDR2, and FR3 regions. The first three sites were conserved in >97% of subclones in and across disease events, whereas the FR3 site was conserved in 95.5% of the diagnostic subclones and in ~80% of the relapsed and transformed populations. No subclones with loss of all four sites were detected for Patient 2. Patient 5 samples were taken from different anatomical sites with tumor populations acquiring distinct N-gly motifs, suggesting an early divergence in tumor evolution. Despite this, a minor population of motif positive clones are shared, suggesting a trafficking ability of subclones. Subclones with motifs made up ≥99% of the total tumor count, highlighting the motif as a feature of the tumor bulk, with motif negative clones representing a minor population. These negative clones are presumably lost during disease progression as they not shared between events. Evolutionary analysis revealed no additional sites are gained as motif positive clones expand while rare negative subclones cannot reacquire sites and do not undergo further diversification.
Conclusion: We report for the first time that acquired N-gly motif sites are a clonal feature in FL disease as seen through their conservation both in the heterogeneous subclonal population and the overall tumor mass. The sites are also retained in progression-associated subclones while rare motif-negative subclones disappear. This suggests that although acquisition of additional driver mutations may dampen the tumor's microenvironment dependency, the motifs and added mannoses may retain functional significance at later stages of disease.
The data indicates motifs as being a universal event of the reservoir cell pool responsible for propagating disease episodes. Targeting N-gly sites and their interacting partners may lead to the disruption of an early and vital FL-microenvironment interaction, presumably mediated through the mannose-lectin interaction, reducing relapse rates and progression of disease.
Forconi:Abbvie: Consultancy; Janssen-Cilag: Consultancy. Gribben:Abbvie: Honoraria; Roche: Honoraria; Pharmacyclics: Honoraria; Novartis: Honoraria; Cancer Research UK: Research Funding; Wellcome Trust: Research Funding; Acerta Pharma: Honoraria, Research Funding; TG Therapeutics: Honoraria; NIH: Research Funding; Kite: Honoraria; Janssen: Honoraria, Research Funding; Unum: Equity Ownership; Medical Research Council: Research Funding; Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.